Short video with simple explanation of differences between alpha, beta and gamma radiation
A longer more in depth explanation of alpha, beta and gamma radiation
Most radioactive elements will emit more than one type of radiation, as the EPA graph below illustrates.
EPA List of common radioactive elements and the types of radiation that they emit, showing that most radioactive elements emit more than one kind of radiation.
The following material will go into more depth, regarding alpha, beta and gamma radiation.
ALPHA PARTICLES
From Wikipedia, the free encyclopedia
"Alpha particle
Composition 2 protons, 2 neutrons
Symbol α, α2+, He2+
4.001506179125(62) u
3.727379240(82) GeV/c2
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as He2+or 42He2+ indicating a Helium ion with a +2 charge (missing its two electrons). If the ion gains electrons from its environment, the alpha particle can be written as a normal (electrically neutral) Helium atom 42He.
actual example of alpha, beta and gamma sources, with a pancake style radiation detector
The nomenclature is not well defined, and thus not all high-velocity helium nuclei are considered by all authors as alpha particles. As with beta andgamma rays/particles, the name used for the particle carries some mild connotations about its production process and energy, but these are not rigorously applied.[3] Some science authors may use doubly ionized helium nuclei (He2+) and alpha particles as interchangeable terms. Thus, alpha particles may be loosely used as a term when referring to stellar helium nuclei reactions (for example the alpha processes), and even when they occur as components of cosmic rays. A higher energy version of alphas than produced in alpha decay is a common product of an uncommon nuclear fission result called ternary fission. However, helium nuclei produced by particle accelerators (cyclotrons,synchrotrons, and the like) are less likely to be referred to as "alpha particles".
Alpha particles, like helium nuclei, have a net spin of zero. Due to the mechanism of their production in standard alpha radioactive decay, alpha particles generally have a kinetic energy of about 5 MeV, and a velocity in the vicinity of 5% the speed of light (see discussion below for the limits of these figures in alpha decay).
They are a highly ionizing form of particle radiation, and (when resulting from radioactive alpha decay) have low penetration depth. They are able to be stopped by a few centimeters of air, or by the skin. However, so-called long range alpha particles from ternary fission are three times as energetic, and penetrate three times as far. As noted, the helium nuclei that form 10-12% of cosmic rays are also usually of much higher energy than those produced by nuclear decay processes, and are thus capable of being highly penetrating and able to traverse the human body and also many meters of dense solid shielding, depending on their energy. To a lesser extent, this is also true of very high-energy helium nuclei produced by particle accelerators.
ALPHA PARTICLES 1000 TIMES MORE DANGEROUS THAN BETA OR GAMMA INTERNALLY
When alpha particle emitting isotopes are ingested, they are far more dangerous than their half-life or decay rate would suggest, due to the high relative biological effectiveness of alpha radiation to cause biological damage, after alpha-emitting radioisotopes enter living cells. Ingested alpha emitter radioisotopes (such as transuranics or actinides) are an average of about 20 times more dangerous, and in some experiments up to 1000 times more dangerous, than an equivalent activity of beta emitting or gamma emitting radioisotopes.
Due to the short range of absorption and inability to penetrate the outer layers of skin, alpha particles are not, in general, dangerous to life unless the source is ingested or inhaled, in which case they become extremely dangerous.[5] Because of this high mass and strong absorption, if alpha-emitting radionuclides do enter the body (upon being inhaled, ingested, or injected, as with the use of Thorotrast for high-quality X-ray images prior to the 1950s), alpha radiation is the most destructive form of ionizing radiation.
It is the most strongly ionizing, and with large enough doses can cause any or all of the symptoms of radiation poisoning. It is estimated that chromosome damage from alpha particles is anywhere from 10 to 1000 times greater than that caused by an equivalent amount of gamma or beta radiation, with the average being set at 20 times.
The powerful alpha emitter polonium-210 (a milligram of 210Po emits as many alpha particles per second as 4.215 grams of 226Ra) is suspected of playing a role in lung cancer andbladder cancer related to tobacco smoking.[6] 210Po was used to kill Russian dissident and ex-FSB officer Alexander V. Litvinenko in 2006.[7]
SOURCES OF ALPHA PARTICLES
A physicist observes alpha particles from the decay of a polonium source in a cloud chamber
ALPHA RADIATION COMES FROM DECAYING RADIOACTIVE ELEMENTS
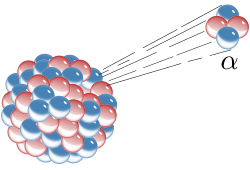
The most well-known source of alpha particles is alpha decay of heavier (> 106 uatomic weight) atoms. When an atom emits an alpha particle in alpha decay, the atom's mass number decreases by four due to the loss of the four nucleons in the alpha particle. The atomic number of the atom goes down by exactly two, as a result of the loss of two protons – the atom becomes a new element. Examples of this sort of nuclear transmutation are when uranium becomes thorium, or radium becomes radon gas, due to alpha decay.
ALPHA PARTICLES COME FROM LARGER RADIOACTIVE NUCLEI
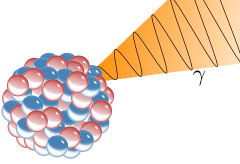
Alpha particles are commonly emitted by all of the larger radioactive nuclei such as uranium, thorium, actinium, and radium, as well as the transuranic elements. Unlike other types of decay, alpha decay as a process must have a minimum-size atomic nucleus that can support it. The smallest nuclei that have to date been found to be capable of alpha emission are the lightest nuclides of tellurium (element 52), with mass numbers between 106 and 110. The process of alpha decay sometimes leaves the nucleus in an excited state, wherein the emission of a gamma ray then removes the excess energy.
ENERGY AND ABSORPTION
The energy of the alpha emitted in alpha decay is mildly dependent on the half-life for the emission process, with many orders of magnitude differences in half-life being associated with energy changes of less than 50% (see alpha decay). The energy of alpha particles emitted varies, with higher energy alpha particles being emitted from larger nuclei, but most alpha particles have energies of between 3 and 7 MeV (mega-electron-volts), corresponding to extremely long and extremely short half-lives of alpha-emitting nuclides, respectively.
This energy is a substantial amount of energy for a single particle, but their high mass means alpha particles have a lower speed (with a typical kinetic energy of 5 MeV; the speed is 15,000 km/s, which is 5% of the speed of light) than any other common type of radiation (β particles, neutrons, etc.)[4] Because of their charge and large mass, alpha particles are easily absorbed by materials, and they can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 micrometres, equivalent to a fewcells deep)."
http://en.wikipedia.org/wiki/Alpha_particle
See also;
http://agreenroad.blogspot.com/2012/04/alpha-radiation-dangers-polonium-radon.html
Polonium 210 Radioactive Alpha Emittter - Widely Used In Government Assassinations Such As Arafat; via @AGreenRoad
http://agreenroad.blogspot.com/2013/10/polonium-210-radioactive-alpha-emittter.html
Can Geiger Counters Detect Plutonium? via @AGreenRoad
BETA PARTICLES
Beta particle
From Wikipedia, the free encyclopedia
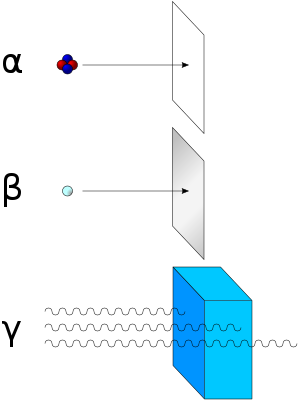
Alpha radiation consists of helium nuclei and is readily stopped by a sheet of paper. Beta radiation, consisting of electrons or positrons, is halted by an aluminum plate. Gamma radiation is dampened by lead.
Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles emitted are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay. They are designated by the Greek letter beta (β). There are two forms of beta decay, β− and β+, which respectively give rise to the electron and the positron.[1]
β− decay (electron emission)
Beta decay. A beta particle (in this case a negative electron) is shown being emitted by a nucleus. An antineutrino is always emitted along with electrons (not shown). Insert: in the decay of free neutron, a proton, an electron (negative beta ray), andelectron antineutrino are produced
An unstable atomic nucleus with an excess of neutronsmay undergo β− decay, where a neutron is converted into a proton, an electron and an electron-type antineutrino (the antiparticle of the neutrino):n → p + e− + νe
This process is mediated by the weak interaction. The neutron turns into a proton through the emission of avirtual W− boson. At the quark level, W− emission turns a down-type quark into an up-type quark, turning a neutron (one up quark and two down quarks) into a proton (two up quarks and one down quark). The virtual W− boson then decays into an electron and an antineutrino.
Beta decay commonly occurs among the neutron-rich fission byproducts produced in nuclear reactors. Free neutrons also decay via this process. Both of these processes contribute to the copious numbers of beta rays and electron antineutrinos produced by fission reactor fuel rods.
β+ decay (positron emission)
Unstable atomic nuclei with an excess of protons may undergo β+ decay, also called positron decay, where a proton is converted into a neutron, a positron and an electron-type neutrino:p → n + e+ + νe
Beta plus decay can only happen inside nuclei when the absolute value of the binding energy of the daughter nucleus is greater than that of the mother nucleus, i.e., the daughter nucleus is a lower-energy state
.
Interaction with other matter
Blue Cherenkov radiation light being emitted from a TRIGA reactor pool is due to high speed beta particles traveling faster than the speed of light (phase velocity) in water (which is 75% of the speed of light in vacuum).
Of the three common types of radiation given off by radioactive materials, alpha, beta and gamma, beta has the medium penetrating power and the medium ionising power. Although the beta particles given off by different radioactive materials vary in energy, most beta particles can be stopped by a few millimeters of aluminium. Being composed of charged particles, beta radiation is more strongly ionising than gamma radiation. When passing through matter, a beta particle is decelerated by electromagnetic interactions and may give off bremsstrahlung x-rays.
In water, beta radiation from many nuclear fission products typically exceeds the speed of light in that material (which is 75% that of light in vacuum),[2]and thus generates blue Cherenkov radiation when it passes through water. The intense beta radiation from the fuel rods of pool-type reactors can thus be visualized through the transparent water that covers and shields the reactor (see illustration at right).
Detection and measurement
The ionising or excitation effects of beta particles on matter are the fundamental processes by which radiometric detection instruments detect and measure beta radiation. The ionisation of gas is used in ion chambers, and Geiger-Muller counters, and the excitation of scintillators is used inscintillation counters.
BETA PARTICLE NEGATIVE HEALTH EFFECTS
Beta particles are able to penetrate living matter to a certain extent and can change the structure of struck molecules. In most cases such change can be considered as damage with results possibly as severe as cancer and death. If the struck molecule is DNA it can show a spontaneous mutation."
Source; http://en.wikipedia.org/wiki/Beta_particle
See also;
Beta Radiation Burns In Animals, Plants And Humans, From Medical Radiation, Nuclear Bombs, Nuclear Accidents, And More
http://agreenroad.blogspot.com/2014/08/beta-radiation-burns-in-animals-and.html
See also;
Beta Radiation Burns In Animals, Plants And Humans, From Medical Radiation, Nuclear Bombs, Nuclear Accidents, And More
http://agreenroad.blogspot.com/2014/08/beta-radiation-burns-in-animals-and.html
GAMMA RAYS
Illustration of an emission of a gamma ray (γ) from an atomic nucleus.
Gamma rays are emitted during nuclear fission in nuclear explosions.
DEFINITION
Gamma radiation, also known as gamma rays,and denoted by the Greek letter γ, refers to electromagnetic radiation of extremely high frequency and therefore high energy per photon. Gamma rays are ionizing radiation, and are thus biologically hazardous. They are classically produced by the decay from high energy states of atomic nuclei (gamma decay), but are also created by other processes. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium. Villard's radiation was named "gamma rays" by Ernest Rutherford in 1903.
Natural sources of gamma rays on Earth include gamma decay from naturally occurring radioisotopes, and secondary radiation from atmospheric interactions with cosmic ray particles. Rare terrestrial natural sources produce gamma rays that are not of a nuclear origin, such as lightning strikes and terrestrial gamma-ray flashes. Additionally, gamma rays are also produced by a number of astronomical processes in which very high-energy electrons are produced, that in turn cause secondary gamma rays via bremsstrahlung, inverse Compton scattering andsynchrotron radiation. However, a large fraction of such astronomical gamma rays are screened by Earth's atmosphere and can only be detected by spacecraft. (They do not reach Earth's surface.)
Gamma rays typically have frequencies above 10 exahertz (or >1019 Hz), and therefore have energies above 100 keV and wavelengths less than 10 picometers (less than the diameter of an atom). However, this is not a hard and fast definition, but rather only a rule-of-thumb description for natural processes.
RADIOACTIVE DECAY
Gamma rays from radioactive decay are defined as gamma rays no matter what their energy, so that there is no lower limit to gamma energy derived from radioactive decay. Gamma decay commonly produces energies of a few hundred keV, and almost always less than 10 MeV.
Thus, gamma rays are now usually distinguished by their origin: X-raysare emitted by definition by electrons outside the nucleus, while gamma rays are emitted by the nucleus.[6][8][9][10]
SHIELDING FROM GAMMA RADIATION
Shielding from gamma rays requires large amounts of mass, in contrast to alpha particles which can be blocked by paper or skin, and beta particles which can be shielded by foil. Gamma rays are better absorbed by materials with high atomic numbers and high density, although neither effect is important compared to the total mass per area in the path of the gamma ray. For this reason, a lead shield is only modestly better (20–30% better) as a gamma shield, than an equal mass of another shielding material such as aluminium, concrete, water or soil; lead's major advantage is not in lower weight, but rather its compactness due to its higher density. Protective clothing, goggles and respirators can protect from internal contact with or ingestion of alpha or beta emitting particles, but provide no protection from gamma radiation from external sources.
The higher the energy of the gamma rays, the thicker the shielding made from the same shielding material is required. Materials for shielding gamma rays are typically measured by the thickness required to reduce the intensity of the gamma rays by one half (the half value layer or HVL). For example gamma rays that require 1 cm (0.4″) of lead to reduce their intensity by 50% will also have their intensity reduced in half by 4.1 cm of granite rock, 6 cm (2½″) of concrete, or 9 cm (3½″) of packed soil.
In a nuclear power plant, shielding can be provided by steel and concrete in the pressure and particle containment vessel, while water provides a radiation shielding of fuel rods during storage or transport into the reactor core. The loss of water or removal of a "hot" fuel assembly into the air would result in much higher radiation levels than when kept under water.
Gamma rays from radioactive gamma decay are produced alongside other forms of radiation such as alpha or beta, and are produced after the other types of decay occur. The mechanism is that when a nucleus emits an α or β particle, the daughter nucleus is usually left in an excited state. It can then move to a lower energy state by emitting a gamma ray photon, in the same way that an atomic electron can jump to a lower energy state by emitting a light ray photon. Emission of a gamma ray from an excited nuclear state typically requires only 10−12 seconds, and is thus nearly instantaneous.
An emitted gamma ray from any type of excited state may transfer its energy directly to one of the most tightly bound electrons causing it to be ejected from the atom, a process termed the photoelectric effect (it should not be confused with the internal conversion process, in which no real gamma ray photon is produced as an intermediate particle).
DECAY OF COBALT 60
Gamma rays, X-rays, visible light, and radio waves are all forms ofelectromagnetic radiation. The only difference is the frequency and hence the energy of those photons. Gamma rays are generally the most energetic of these, although broad overlap with X-ray energies occurs. An example of gamma ray production follows:
First 60Co decays to excited 60Ni by beta decay by emission of an electron of 0.31 MeV. Then the excited 60Ni drops down to the ground state (see nuclear shell model) by emitting two gamma rays in succession (1.17 MeV then 1.33 MeV).
DECAY OF AMERICIUM 241
Another example is the alpha decay of 241Am to form 237Np; this alpha decay is accompanied by gamma emission. In some cases, the gamma emission spectrum for a nucleus (daughter nucleus) is quite simple, (e.g. 60Co/60Ni) while in other cases, such as with (241Am/237Np and 192Ir/192Pt), the gamma emission spectrum is complex, revealing that a series of nuclear energy levels can exist. The fact that an alpha spectrum can have a series of different peaks with different energies reinforces the idea that several nuclear energy levels are possible..
Gamma radiation, like X-radiation, can be produced by a variety of phenomena. When high-energy gamma rays, electrons, or protons bombard materials, the excited atoms within emit characteristic "secondary" gamma rays, which are products of the temporary creation of excited nuclear states in the bombarded atoms (such transitions form a topic in nuclear spectroscopy). Such gamma rays are produced by the nucleus, but not as a result of nuclear excitement from radioactive decay.
NEGATIVE HEALTH EFFECTS
All ionizing radiation causes similar damage at a cellular level... Gamma rays and neutrons are more penetrating, causing diffuse damage throughout the body (e.g. radiation sickness, cell's DNA damage, cell death due to damaged DNA, increasing incidence of cancer) rather than burns. External radiation exposure should also be distinguished from internal exposure, due to ingested or inhaled radioactive substances, which, depending on the substance's chemical nature, can produce both diffuse and localized internal damage.
The retina too is damaged by exposure to gamma irradiation, showing decreased DNA and RNA contents, with some increase in alkaline phosphatase activity [18]
BODY RESPONSE
When gamma radiation breaks DNA molecules, a cell may be able to repair the damagedgenetic material, within limits. However, a study of Rothkamm and Lobrich has shown that this repair process works well after high-dose exposure but is much slower in the case of a low-dose exposure.[19]
RISK ASSESSMENT
The natural outdoor exposure in Great Britain ranges from 2 to 4 nSv/h (nanosieverts perhour).[20] Natural exposure to gamma rays is about 1 to 2 mSv per year, and the average total amount of radiation received in one year per inhabitant in the USA is 3.6 mSv.[21] There is a small increase in the dose, due to naturally occurring gamma radiation, around small particles of high atomic number materials in the human body caused by the photoelectric effect.[22]
MEDICAL EXPOSURE
By comparison, the radiation dose from chest radiography (about 0.06 mSv) is a fraction of the annual naturally occurring background radiation dose.[23] A chest CT delivers 5 to 8 mSv. A whole-body PET/CT scan can deliver 14 to 32 mSv depending on the protocol.[24] The dose fromfluoroscopy of the stomach is much higher, approximately 50 mSv (14 times the annual yearly background).
ACUTE SINGLE EXPOSURE DOSE
An acute full-body equivalent single exposure dose of 1 Sv (1000 mSv) causes slight blood changes, but 2.0–3.5 Sv (2.0–3.5 Gy) causes very severe syndrome of nausea, hair loss, and hemorrhaging, and will cause death in a sizable number of cases—-about 10% to 35% without medical treatment. A dose of 5 Sv[25] (5 Gy) is considered approximately the LD50 (lethal dose for 50% of exposed population) for an acute exposure to radiation even with standard medical treatment. A dose higher than 5 Sv (5 Gy) brings an increasing chance of death above 50%.
Above 7.5–10 Sv (7.5–10 Gy) to the entire body, even extraordinary treatment, such as bone-marrow transplants, will not prevent the death of the individual exposed (see Radiation poisoning). (Doses much larger than this may, however, be delivered to selected parts of the body in the course of radiation therapy.)
For low dose exposure, for example among nuclear workers, who receive an average yearly radiation dose of 19 mSv, the risk of dying from cancer (excluding leukemia) increases by 2 percent. For a dose of 100 mSv, the risk increase is 10 percent. By comparison, risk of dying from cancer was increased by 32 percent for the survivors of the atomic bombing of Hiroshima and Nagasaki.[26]"
Source; http://en.wikipedia.org/wiki/Gamma_particle
End
Alpha Particles, Beta Particles and Gamma Radiation Explained
http://agreenroad.blogspot.com/2014/08/alpha-particles-beta-particles-and.html
More articles at;
Teaching the Science Of Sustainable Health And Success
http://agreenroad.blogspot.com/2014/08/alpha-particles-beta-particles-and.html
More articles at;
Individual Radioactive Elements/Isotopes, USA Radiation Exposure Prevention and Reversal, Music
Teaching the Science Of Sustainable Health And Success
(1,000 + Creative Commons Videos)
http://tinyurl.com/agryoutube
Table of Contents
http://tinyurl.com/agrindex
2,000 + Videos And Articles
http://tinyurl.com/agryoutube
Table of Contents
http://tinyurl.com/agrindex
2,000 + Videos And Articles
Website; www.agreenroadproject.org
(1,000 + Creative Commons Youtube Videos)
http://tinyurl.com/agryoutube
Table Of Contents
http://tinyurl.com/agrindex
2,000 + Videos And Articles
http://tinyurl.com/agryoutube
Table Of Contents
http://tinyurl.com/agrindex
2,000 + Videos And Articles
Links To Other Notable Organizations; via @AGreenRoad
http://agreenroad.blogspot.com/2014/04/links-to-other-notable-organizations.html
Email Contact; agreenroad at gmail.com
What works for seven future generations, without causing harm?
Email Contact; agreenroad at gmail.com
What works for seven future generations, without causing harm?







Post a Comment