Did plutonium exist in Nature in the recent past? The answer is a realistic NO. Are there 'natural' fission reactors on Earth? The answer is a realistic NO. We have to go back 1.7 billion years to find any plutonium or fission reactions on Earth, and then it occurred only in one place on Earth, and no where else, and at no other time.
As you can see from the chart above, inhaled plutonium hot particles are 115,000 times worse than just about any other radioactive element in terms of negative health effects. So where do these plutonium hot particles come from? Are they 'natural'?
As you can see from the chart above, inhaled plutonium hot particles are 115,000 times worse than just about any other radioactive element in terms of negative health effects. So where do these plutonium hot particles come from? Are they 'natural'?
UNDERGROUND 'NATURAL FISSION'
According to Wikipedia; "Trace amounts of at least three plutonium isotopes (plutonium 239 in a few parts per trillion, and its decay products are 'naturally' found ONLY in some concentrated ores of uranium,[43] at the ancient but dead natural nuclear fission reactor in Oklo, Gabon.[44]
Oklo is the only known location for this in the world and consisted of self-sustaining nuclear fission reactions that took place approximately 1.7 billion years ago, and ran for a few hundred thousand years, averaging 100 kW of thermal power during that time.[2][3]
http://en.wikipedia.org/wiki/Natural_nuclear_fission_reactor
VanneV October 7, 2014 Oklo reactor stopped fissioning one and a half BILLION years ago. And there is no chance that it could again in nature today or anywhere else.: “…Such a reactor could not exist today, because too much of the Earth's natural U-235 has decayed… but a billion and a half years ago, there was enough of it around to make the idea plausible….”
For practical intents and purposes, there is no exposure or measurable amount of uranium, plutonium, cesium or strontium radiation that can be found in anyone globally due to Oklo. Plutonium, Tritium Cesium, and Strontium are not things that come out of 'natural' uranium/thorium deposits around the world. Why not? With the exception of a few thorium sand beaches, people are not exposed to plutonium, cesium, strontium, uranium or thorium because these deposits are underground, and far away from human contact, bound up in rock deposits.
However, that being said, radioactive radon gas is an issue in some homes, as it is a decay product from underground radioactive elements.
UNDERGROUND NUCLEAR REACTION HAPPENED 1.7 BILLION YEARS AGO
Note; this totally natural and underground Oklo nuclear reaction happened 1.7 BILLION years ago, way before there was any human life on Earth. This underground, invisible and undetectable nuclear reaction stopped way before human living beings inhabited the planet.
So for all intents and purposes, there has been no 'natural' fission happening and there has been no plutonium has been around since humans walked the Earth, contrary to what pro nuclear apologists claim. All of the plutonium generated by this underground reaction has decayed away. If any is left, that miniscule amount is still found underground, only in the one location, at Oklo, Africa, where almost no one lives.
Wikipedia has another entry for plutonium; "Primordial plutonium-244 has a relatively long half-life of about 80 million years.[46] These trace amounts of 239Pu originated in the following fashion: On rare occasions, 238U undergoes spontaneous fission, and in the process, the nucleus emits one or two free neutrons with some kinetic energy. When one of these neutrons strikes the nucleus of another 238U atom, it is absorbed by the atom, which becomes 239U. With a relatively short half-life, U-239 decays to neptunium-239 (239Np), and then 239Np decays into 239Pu.
Since the relatively long-lived isotope plutonium-240 occurs in the decay chain of plutonium-244 it should also be present, albeit 10,000 times rarer still. Finally, exceedingly small amounts of plutonium-238, attributed to the extremely rare double beta decay of uranium-238, have been found in natural uranium samples.[47]"
http://en.wikipedia.org/wiki/Plutonium
A person would have to dig around in or mine uranium deposits such as Oklo in Africa, to be exposed to radioactive dust, particles and ingest things like uranium, radon or thorium. Miners involved with the nuclear industry can and do get exposed to radioactive dust and then get diseases and cancers from this. But the average person is not exposed to radioactive dust or particles unless there is a uranium/thorium mind or a nuclear facility emitting these things upwind. For all practical intents and purposes, there is no natural fission happening on Earth at this point. Maybe a billion years ago, yes, but not now.
http://en.wikipedia.org/wiki/Plutonium
A person would have to dig around in or mine uranium deposits such as Oklo in Africa, to be exposed to radioactive dust, particles and ingest things like uranium, radon or thorium. Miners involved with the nuclear industry can and do get exposed to radioactive dust and then get diseases and cancers from this. But the average person is not exposed to radioactive dust or particles unless there is a uranium/thorium mind or a nuclear facility emitting these things upwind. For all practical intents and purposes, there is no natural fission happening on Earth at this point. Maybe a billion years ago, yes, but not now.
ANY MEASURABLE PLUTONIUM FROM OKLO, DISAPPEARED 200 MILLION YEARS AGO
With an 80 million year half life, all of the measurable plutonium that happened from the fission process below ground in Oklo, disappeared in 800 million years. This is still a couple hundred million years before people even walked on the planet. Bottom line, anyone who says that large amounts of plutonium were lying around all over the place naturally before the atomic age covered the Earth with it is either lying or trying to deceive someone into believing that plutonium is 'safe' or 'natural' somehow, via the hormesis theory.
Via VanneV November 26, 2014 “…Nearly all plutonium is man-made. It does occur naturally in very small amounts in a type of ore called pitchblende. Once the main source of uranium and radium, pitchblende contains one part per trillion of natural plutonium. “Plutonium predominantly emits alpha particles—a type of radiation that does not penetrate and has a short range so it is easy to contain. But plutonium can be long-lived. It can deposit in the bones and lungs, and could increase an individual’s cancer risk. Therefore, exposure limits are set very low….”
IS THERE PLUTONIUM IN PITCHBLENDE?
There are 6e11 atoms of plutonium in every 238 grams of uranium in pitchblende. One part per trillion is still a significant number of atoms.
Via hbjon October 17, 2014 "there are different types of uranium that produce plutonium in different quantities. In the rare occurrence that SF happens inside the ore, it is even a rarer occurrence that an atom of Pu will be produced. The reason is ore has been cool for so long and the instances of SF is approaching zero. Within pitchblende there are not atoms of U238 right next to each other."
Via hbjon October 17, 2014 "there are different types of uranium that produce plutonium in different quantities. In the rare occurrence that SF happens inside the ore, it is even a rarer occurrence that an atom of Pu will be produced. The reason is ore has been cool for so long and the instances of SF is approaching zero. Within pitchblende there are not atoms of U238 right next to each other."
What hb is pointing at is that uranium ore contains mostly rock and a very small amount of uranium.
"Uranium ores are normally processed by grinding the ore materials to a uniform particle size and then treating the ore to extract the uranium by chemical leaching. The milling process commonly yields dry powder-form material consisting of natural uranium, "yellowcake," which is sold on the uranium market as U3O8."
http://en.wikipedia.org/wiki/Uranium_mining
Theoretically, a neutron in pitchblende can be captured in U238 and eventually transmute to a Pu239 but the odds of that happening are so remote that it is hard to even calculate, because these uranium atoms are scattered throughout rocks and water, making it even less likely. The rocks containing uranium ore have to be ground up into fine particles and then treated with chemicals to get the uranium out of it. Uranium ore or (pitchblende) is not like gold, where you find large nuggets of it laying around.
"Pitchblende" is composed of many things, including lead, which all radioactive elements decay into. hbjon October 17, 2014 "Because of its age, the uranium has become so contaminated with decay products, that any neutron releases are absorbed by them. This will produce trace quantities of actinium and protactinium, among other isotopes along the decay chain of U238. The "signals" that are detected should be entirely alpha, beta, and gamma radiation. All the natural plutonium on earth couldn't produce enough power to turn on a light bulb."
Angela_R October 18, 2014 "Plutonium in pitchblende? For many decades we've had 'atoms for peace'. The (nuclear) waste has been distributed in many ways. I seem to recall that it is used in roads, in building materials, dumped as fill, distributed from the sky in fallout from depleted uranium. I've also heard that it is even in kitchen utensils...
In other words, why look for a needle in a haystack, when there are massive amounts of radioactive plutonium, uranium, and other man made radioactive waste products being dumped, vented and spewed into the waterways of the Earth. The amount of nuclear waste material dumped into air, water and ground is so vast, that there is no way to compare it to any 'natural' process that may happen in pitchblende. Any miniscule amount of plutonium created in this way is bound up in rocks, so it does not float around in the air, as does the plutonium emitted from man made sources.
In other words, why look for a needle in a haystack, when there are massive amounts of radioactive plutonium, uranium, and other man made radioactive waste products being dumped, vented and spewed into the waterways of the Earth. The amount of nuclear waste material dumped into air, water and ground is so vast, that there is no way to compare it to any 'natural' process that may happen in pitchblende. Any miniscule amount of plutonium created in this way is bound up in rocks, so it does not float around in the air, as does the plutonium emitted from man made sources.
VanneV October 18, 2014 “Plutonium appears at very low concentrations in nature, on the order (of one part in 10^11) in pitchblende, the ore of uranium (U)…. “The isotopes of uranium decay primarily by alpha-particle emission, but there is also a process called "spontaneous fission" that occasionally competes with alpha decay. “In spontaneous fission, the nucleus splits (‘fissions’) and additional neutrons are released. There is a possibility that these released neutrons are absorbed (captured) by another U-238 nucleus. If this occurs, it triggers a process that produces Pu-239 in a manner similar to that discussed above. Thus, we have plutonium produced naturally in the environment (admittedly in trace quantities). This reaction has been going on since the creation of the Earth. “In 1971, Darlene Hoffman of Los Alamos National Laboratory discovered trace quantities of another isotope of plutonium in the environment. Pu-244 was found in Precambrian Age phosphate from southern California. This isotope of plutonium had a radioactive half-life of 80 million years. Scientists have postulated that, because of its long radioactive half-life, this isotope has existed since the creation of Earth about 4.5 billion years ago. …”
VanneV October 18, 2014 "Plutonium isn't found in all pitchblend, but very rarely. “An engineer can use the formula below to calculate the approximate number of plutonium atoms.
n = 2.5 × 10^21 ×m . In this formula, N is the approximate number of plutonium atoms, and M is the mass of the plutonium in grams.
"Suppose a nuclear engineer wants to calculate how many plutonium atoms are in 2 grams of plutonium.
n = 2.5 × 10^21 × 2
n = 5 × 10^21
"There are 5 × 10^21 plutonium atoms in 2 grams
razzz October 18, 2014 "Who said all Pu was man made? If ManBearPig is correct that Pu is created in natural uranium deposits either by chance of a slowed neutron hitting its mark or enough time and random water switching the U reaction on and off, can you imagine how much new Pu has been created and how much is still being created amongst the Daiichi melts? I guarantee you that the Fukus' melts have created more new Pu than all of earth's natural uranium surface deposits."
VanneV October 18, 2014 "At best, there are only microscopic amounts and only 1 or 2 isotopes. I could only find 2 places where they found natural plutonium, and there are rumors that the mines were salted. “…Microscopic amounts of Plutonium are made by neutron capture by Uranium, and yet occur naturally….”
http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Descriptive_Chemistry/f-Block_Elements/The_Actinides
VanneV October 18, 2014 From Google: "Trace amounts of at least three plutonium isotopes (plutonium-238, 239, and 244) can be found in nature. Small traces of plutonium-239, a few parts per trillion, and its decay products are naturally found in some concentrated ores of uranium, such as the natural nuclear fission reactor in Oklo, Gabon." And nothing was found in nature until almost 30 years after plutonium was created artificially.
VanneV October 18, 2014 “…Exceedingly small amounts of Pu-238, attributed to the extremely rare double-beta decay of U-238, have been found in natural uranium samples. Plutonium was most likely formed by neutron activation of natural U-238 at the Oklo natural reactor, but if formed it has long since decayed away….”
http://periodic.lanl.gov/94.shtml
PLUTONIUM IS AN ARTIFICIAL ELEMENT WITH NO KNOWN POSITIVE ROLE IN THE HUMAN BODY
VanneVOctober 18, 2014 “…Plutonium (Pu) is an artificial element, except for trace quantities of primordial 244Pu, and thus a standard atomic mass cannot be given. Like all artificial elements, it has no stable isotopes.
It was synthesized long before being found in nature, the first isotope synthesized being 238Pu in 1940. Twenty plutonium radioisotopes have been characterized. The most stable are Pu-244, with a half-life of 80.8 million years, Pu-242, with a half-life of 373,300 years, and Pu-239, with a half-life of 24,110 years. All of the remaining radioactive isotopes have half-lives that are less than 7,000 years. This element also has eight meta states, though none is very stable; all meta states have half-lives of less than one second….”
“…Plutonium has no naturally occurring isotopes….”
It was synthesized long before being found in nature, the first isotope synthesized being 238Pu in 1940. Twenty plutonium radioisotopes have been characterized. The most stable are Pu-244, with a half-life of 80.8 million years, Pu-242, with a half-life of 373,300 years, and Pu-239, with a half-life of 24,110 years. All of the remaining radioactive isotopes have half-lives that are less than 7,000 years. This element also has eight meta states, though none is very stable; all meta states have half-lives of less than one second….”
THERE IS NO 'NATURAL' PLUTONIUM LYING AROUND
Some pro nuclear apologists even go so far as to claim that because plutonium is 'primordial' and 'natural', it is safe and 'normal' to be around. They ask the question that says; if "there is no safe dose", can you get a deadly dose from "trace amounts" of Pu?
The only problem with this theory and question, is that for all practical purposes and intents, 'natural' plutonium cannot be found, because the amounts are so small, so buried, so hard to find, so difficult to get to such as in the Oklo deposits, that for all realistic and practical medical purposes, plutonium did not exist in the human environment before the atomic age and humans created it in the lab. The amounts (if they even exist) are so hard to find, so minute in amount, and so locked up in rocks, that the pro nuclear scientists did not even find it until 30 years AFTER they synthetically created plutonium in a lab.
The only problem with this theory and question, is that for all practical purposes and intents, 'natural' plutonium cannot be found, because the amounts are so small, so buried, so hard to find, so difficult to get to such as in the Oklo deposits, that for all realistic and practical medical purposes, plutonium did not exist in the human environment before the atomic age and humans created it in the lab. The amounts (if they even exist) are so hard to find, so minute in amount, and so locked up in rocks, that the pro nuclear scientists did not even find it until 30 years AFTER they synthetically created plutonium in a lab.
Whatever 'primordial' plutonium exists in nano particle amounts, is only associated with uranium deposits and is locked into those rocks. No one was stupid enough to dig up these very toxic and deadly elements before the atomic age and then grind them into a powder in order to extract the uranium via chemical processes. The parts per TRILLION amount of plutonium found in 1 or 2 'primordial' uranium deposits somewhere in the wilds of Africa where no one lived, did not register on any medical tests and no one died from it, unless they dug it up and played with it, as Madame Curie did.
If a person were to read the Wiki entry about spontaneous fission, it would be easy to assume that 'natural' fission is happening all around, every day, in every home, city and neighborhood, when that is not the case. Natural 'spontaneous fission' happens underground in thorium or uranium rock deposits.
http://en.wikipedia.org/wiki/Spontaneous_fission
They only way to detect this 'natural' decay process happening underground is by the radon gas being emitted from the ground, which comes into peoples basements if they build on top of this type of geological formation. If the uranium containing rocks are on the surface, the radiation emitting from those rocks can be detected with a simple Geiger counter. What the radiation detector is detecting is the uranium, not any plutonium.
Plutonium, strontium, cesium and tritium, plus 2,000 other man made radioactive elements are not released by these uranium containing ore rocks. Synthetic man made elements, isotopes and actinides are created by burning concentrated nuclear fuel inside of a cyclotron, a nuclear reactor or a breeder reactor, where these artificial elements are then produced in huge quantities.
If a person were to read the Wiki entry about spontaneous fission, it would be easy to assume that 'natural' fission is happening all around, every day, in every home, city and neighborhood, when that is not the case. Natural 'spontaneous fission' happens underground in thorium or uranium rock deposits.
http://en.wikipedia.org/wiki/Spontaneous_fission
They only way to detect this 'natural' decay process happening underground is by the radon gas being emitted from the ground, which comes into peoples basements if they build on top of this type of geological formation. If the uranium containing rocks are on the surface, the radiation emitting from those rocks can be detected with a simple Geiger counter. What the radiation detector is detecting is the uranium, not any plutonium.
Plutonium, strontium, cesium and tritium, plus 2,000 other man made radioactive elements are not released by these uranium containing ore rocks. Synthetic man made elements, isotopes and actinides are created by burning concentrated nuclear fuel inside of a cyclotron, a nuclear reactor or a breeder reactor, where these artificial elements are then produced in huge quantities.
PARTS PER TRILLION PLUTONIUM IS BOUND UP IN URANIUM DEPOSITS UNDERGROUND, SAFELY HELD AWAY FROM ALL HUMAN CONTACT
The miniscule atoms of plutonium inside of a few uranium deposits globally, such as at Okla, can only be measured in parts per trillion or less, and not all pitchblende deposits contain plutonium. Plutonium inside uranium rocks is so hard to find, it takes special nuclear physics lab equipment to even detect it. No Geiger counter will find it or be able to measure it. Effectively, before the atomic age, no human exposure to plutonium was possible, and plutonium, strontium, cesium, and tritium contamination was not observable or detectable in humans before the atomic age.
INDIAN CULTURES KEPT A TABOO ON ALL AREAS WITH URANIUM ROCKS, DID NOT DISTURB OR GO NEAR THEM
In addition to the above, uranium or thorium deposits were widely made taboo by indigenous tribes globally. These 'hot' rocks or sands were called 'bad medicine', to be avoided at all costs by indigenous peoples all around the world. The advice to not go near or disturb uranium/thorium deposits, sands or soils is still valid and good advice today. Just because radon, uranium or thorium is 'natural' does not make it safe.
American Indians and indigenous peoples around the globe were told by their shamans and chiefs not to disturb the soil or rocks where uranium was present. People were told to avoid going into these areas. These ancient wise people KNEW 'natural' uranium deposits were dangerous and so they made it taboo to be around any soil or rocks containing uranium.
Only the nuclear scientists and hormesis theory hucksters selling quack radiation cures, or those bent on making nuclear bombs have violated this taboo. Humanity is now paying the price for the violation and breaking of a very ancient and very wise taboo. How and why would this taboo be valid today?
http://youtu.be/iFqEb7hUNT8
American Indians and indigenous peoples around the globe were told by their shamans and chiefs not to disturb the soil or rocks where uranium was present. People were told to avoid going into these areas. These ancient wise people KNEW 'natural' uranium deposits were dangerous and so they made it taboo to be around any soil or rocks containing uranium.
Only the nuclear scientists and hormesis theory hucksters selling quack radiation cures, or those bent on making nuclear bombs have violated this taboo. Humanity is now paying the price for the violation and breaking of a very ancient and very wise taboo. How and why would this taboo be valid today?
http://youtu.be/iFqEb7hUNT8
NOW WE ARE IN AN AGE OF MAN MADE FISSION, THERE IS NOTHING 'NATURAL' ABOUT IT
Plutonium was 'discovered' in 1940 through initiating man made and artificial nuclear fission, which then produced artificial and man made radioactive elements in labs, plutonium, tritium, cesium and thousands of other man made radioactive elements. The man made elements are all those elements in the periodic table above uranium (U92)
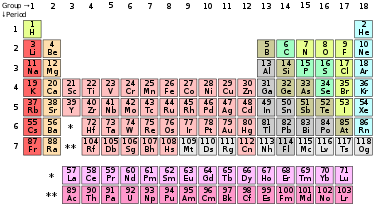
Lawrencium 103Lr
Rutherfordium104 Rf
Dubnium105 Db
Seaborgium 106 Sg
Bohrium 107 Bh
Hassium 108 Hs
Meitnerium 109 Mt
Darmstadtium 110 Ds
Roentgenium 111 Rg
Copernicium 112 Cn
Ununtrium 113 Uut
Flerovium 114 Fl
Ununpentium 115 Uup
Livermorium 116 Lv
Ununseptium 117 Uus
Ununoctium 118 Uuo

Neptunium 93 Np
Plutonium 94 Pu
Americium 95 Am
Curium 96 Cm
Berkelium 97 Bk
Californium 98 Cf
Einsteinium 99 Es
Fermium 100 Fm
Mendelevium 101 Md
Nobelium 102 No
Just because an element is 'natural', does not mean it is good for your health, as some pro nuclear apologists like to claim. To find out more, click on the link and learn more about the dangers of uranium 238.
http://agreenroad.blogspot.com/2013/10/uranium-238-decay-chain-and-negative.html
93 Long Life Radioactive Elements/Isotopes, A Problem For Billions Of Years; via @AGreenRoad
http://agreenroad.blogspot.com/2012/03/93-long-lived-nuclear-elements.html
1,946 Lethal Radioactive Man Made Isotopes Are Created By Nuclear Plants And Atomic Bombs; via @AGreenRoad
http://agreenroad.blogspot.com/2013/10/1946-lethal-radioactive-man-made.html
Fanfare to THE AGE OF FISSION

h.thomas ackermann
http://agreenroad.blogspot.com/2012/03/93-long-lived-nuclear-elements.html
1,946 Lethal Radioactive Man Made Isotopes Are Created By Nuclear Plants And Atomic Bombs; via @AGreenRoad
http://agreenroad.blogspot.com/2013/10/1946-lethal-radioactive-man-made.html
ANYTHING ABOVE URANIUM IN PERIODIC TABLE IS MAN MADE AND ARTIFICIAL, HIGHLY TOXIC AND HIGHLY RADIOACTIVE
The chemist above explains how anything beyond uranium is very radioactive, hard to handle and difficult to make. In other words, the Nature made elements stop at uranium. Everything from there on up is man made and artificial, never having been seen on Earth before. And as we learned from the video before this one, even 'natural' uranium is deadly dangerous.
AGE OF MAN MADE FISSION; WILL HUMANITY LIVE THROUGH IT?
These man made heavy metal and radioactive artificial elements have been concentrated and produced in in great quantities (tens to hundreds of thousands of tons) by weapons labs and nuclear reactors due to nuclear scientists intent on being masters of fission power. The sun works via fusion power, not fission. There is nothing 'natural' about all of these artificial elements that do nothing but kill, destroy and harm all life and genetic codes of all life on the planet.
Fanfare to THE AGE OF FISSION

h.thomas ackermann
As Thomas so eloquently puts it, humans started from the Stone Age, Bronze Age, Iron Age, Age of Enlightenment, The Middle Ages, The Industrial Age and now we are in the Age Of Fission. Will humanity live through it?
A more complete list of ages and geographical breakdowns
Will humanity make it through this fission age, or will it end up destroying itself with the byproducts of fission, (nuclear bombs, DU, nuclear plant melt downs)?
MAN MADE PLUTONIUM NOW MEASURABLE ALL AROUND THE WORLD AS A HUMAN CREATED RADIOACTIVE AND DEADLY CONTAMINATION
Due to numerous accidents and releases of plutonium via pyrophoric plutonium fires in atomic bomb weapons labs, as well as the 'testing' of 2,400 atomic bombs globally, plus the 'accidental' dropping and exploding of hydrogen bombs where the high explosives went off, crashing plutonium satellites, plutonium is now present in detectable amounts in the global environment as a man made radioactive pollutant and toxic heavy metal. Man made radioactive plutonium has gone all around the world.
MAN MADE RADIOACTIVE CESIUM AND STRONTIUM MEASURABLE IN EVERY HUMAN BEING
Some cesium and strontium is now commonly found in the human body due to the 2,400 atmospheric and underwater nuclear tests that have been carried out, as well as due to a small number of major nuclear accidents, such as Santa Susana, Three Mile Island, Fukushima and Chernobyl. Plutonium may also be found in some locations.
"Most atmospheric and underwater nuclear testing was stopped by the Limited Test Ban Treaty in 1963, which was signed and ratified by the United States, the United Kingdom, the Soviet Union, and other nations. Continued atmospheric nuclear weapons testing since 1963 by non-treaty nations included those by China (atomic bomb test above the Gobi Desert in 1964, hydrogen bomb test in 1967, and follow-on tests), and France (tests as recently as the 1990s). Because it is deliberately manufactured for nuclear weapons and nuclear reactors, plutonium-239 is the most abundant isotope of plutonium by far."[32]
Some pro nuclear folks may even claim that the sun is 'natural' fission. But that is also nothing more than deceptive misleading pro nuclear propaganda.
SUN IS NOT A 'NATURAL' SOURCE OF MAN MADE RADIOACTIVE ELEMENTS
Let's also go into the statement within the video that the sun is a source of natural 'radiation'. First of all the sun is powered by fusion, not fission. Trying to say that man made nuclear radioactive elements are as harmless as the sun's light and infrared rays generated by fusion is like trying to say the moon is made of cheese, because it looks like a piece of cheese.
Wikipedia says that; "the Sun is the star at the center of the Solar System. It is almost perfectly spherical and consists of hot plasma interwoven with magnetic fields.[12][13] It has a diameter of about 1,392,684 km (865,374 mi),[5] around 109 times that of Earth, and its mass (1.989×1030 kilograms, approximately 330,000 times the mass of Earth) accounts for about 99.86% of the total mass of the Solar System.[14] Chemically, about three quarters of the Sun's mass consists of hydrogen, while the rest is mostly helium. The remainder (1.69%, which nonetheless equals 5,600 times the mass of Earth) consists of heavier elements, including oxygen, carbon, neon and iron, among others.[15]
SUN WORKS BY FUSION, NOT FISSION
The Sun formed about 4.6 billion[a] years ago from the gravitational collapse of a region within a large molecular cloud. Most of the matter gathered in the center, while the rest flattened into an orbiting disk that would become the Solar System. The central mass became increasingly hot and dense, eventually initiating fusion in its core.
It is thought that almost all stars form by this process. The Sun is a G-type main-sequence star(G2V) based on spectral class and it is informally designated as a yellow dwarf because its visible radiation is most intense in the yellow-green portion of the spectrum, and although it is actually white in color, from the surface of the Earth it may appear yellow because of atmospheric scattering of blue light.[16]In the spectral class label, G2 indicates itssurface temperature, of approximately 5778 K (5505 °C), and V indicates that the Sun, like most stars, is a main-sequence star, and thus generates its energy by fusion of hydrogen nuclei into helium. In its core, the Sun fuses 620 million metric tons of hydrogen each second.....
Through most of the Sun's life, energy is produced by fusion through a series of steps called the p–p (proton–proton) chain; this process converts hydrogen into helium.[48]
The core is the only region in the Sun that produces an appreciable amount of thermal energy through fusion; 99% of the power is generated within 24% of the Sun's radius, and by 30% of the radius, fusion has stopped nearly entirely.The rest of the star is heated by energy that is transferred outward by radiation from the core to the convective layers just outside. The energy produced by fusion in the core must then travel through many successive layers to the solar photosphere before it escapes into space as sunlight or the kinetic energy of particles.[50][51]"
http://en.wikipedia.org/wiki/Sun
SUN'S NATURAL HEALTH AND LIFE SUPPORTING RADIATION THAT HITS EARTH CONSISTS OF HEAT, LIGHT, INFRARED AND ULTRAVIOLET, NOT MAN MADE HARMFUL AND DEADLY POISONOUS RADIATION
The sun's rays emit health giving light, infrared and ultraviolet rays, which humans, plants and animals have evolved over hundreds of thousands or millions of years to be able to live in harmony with and rely on for such things as photosynthesis, generating Vitamin D in the skin and much more.
Humans cannot live in proximity to man made or even 'natural' radioactive elements and thrive. People who work in uranium mines or who breathe in radon get lung cancer and other diseases. The same applies to ingesting one of hundreds of man made radioactive elements; ALL OF THEM are deadly heavy metals which mimic natural minerals, and they emit DEADLY alpha, beta, gamma, and/or neutron radiation which the sun does not.
The sun can burn the skin if a person is not acclimated to it, but if the sun were toxic to all of life, why are all living things not getting cancer and decaying, being burned, and withering away? Yes, even the sun's rays can cause burning and damage to the skin, but this infrared and ultraviolet and visible light energy can be seen and avoided if one wants to. It is easy to sit in the shade or walk around with clothing, or to go out only after the sun goes down.
If this radiation were as toxic and dangerous as man made radiation is, every living thing on earth would be dead by now.
If this radiation were as toxic and dangerous as man made radiation is, every living thing on earth would be dead by now.
MAN MADE RADIOACTIVE ELEMENTS ARE DEADLY AND INVISIBLE, GET INSIDE LIVING THINGS AND DESTROY GENETIC CODE
By comparison, man made radioactive elements cannot be avoided as they are completely invisible, but super deadly in two ways. radioactive elements are both poisonous heavy metals that mimic natural health giving minerals, but they are also radioactive poisons as well.
Radioactive heavy metal gases and hot particles can be breathed in and cause cancer or genetic diseases. There is no way to avoid this. You cannot find 'shade' or drink/eat 'pure' non radioactive contaminated foods or drinks, because these man made radioactive elements concentrate up the food chain to man and build up internally, until they reach disease causing or lethal levels. The sun's rays may hit you, but they do not build up internally to toxic levels. The sun does not cause anything harmful to concentrate up the food chain to lethal levels.
WHAT IS TAKE AWAY?
There is no 'natural nuclear fission' (not even in the sun) and there is no 'natural man made radioactive element'. Even low doses of radiation from man made radioactive elements can and do cause cancer, genetic diseases, diabetes, ALS, heart disease, leukemia and many more.
Man made radioactive elements are created by a fission process, which results in negative transmutation or decay of elements that ends in lead, which is also a deadly heavy metal, even in just minute quantities. The sun does not do any of this. The sun's rays do not result in decay or negative transmutation of elements. The sun's fusion process does not create heavy metals that ends in lead, which then poisons all of life. If it did this, there would be nothing be lead left on the surface of the planet and nothing could live in this poison metal containing water or soil.
Man made radioactive elements are created by a fission process, which results in negative transmutation or decay of elements that ends in lead, which is also a deadly heavy metal, even in just minute quantities. The sun does not do any of this. The sun's rays do not result in decay or negative transmutation of elements. The sun's fusion process does not create heavy metals that ends in lead, which then poisons all of life. If it did this, there would be nothing be lead left on the surface of the planet and nothing could live in this poison metal containing water or soil.
For all practical, health, medical and global purposes, plutonium is nothing more than a toxic heavy metal that acts as a poison directly within the body, just like lead. Plutonium is also a radioactive cancer initiator in the human body. There is no known positive and health giving use for plutonium, uranium, thorium or radon.
Dog Studies And Inhaled Plutonium Radiation Effects; via @AGreenRoad
http://agreenroad.blogspot.com/2014/01/dog-studies-and-inhaled-plutonium.html
NO NATURAL FISSION AND NO NATURAL PLUTONIUM
There is no 'natural fission' (not even in the sun) and there is no 'natural plutonium'. Even low doses of radiation from radon cause lung cancer. In the same way, low doses of plutonium cause cancer as well. To find out how dangerous plutonium is, click on the following links..
How Dangerous Is 400-6000 Pounds Of Plutonium Nano Particle Dust Liberated By Fukushima? Via @AGreenRoad
http://agreenroad.blogspot.com/2013/08/how-dangerous-is-400-6000-pounds-of.html
Fukushima Released Massive Amounts of Plutonium; It Was Found In Japan, The Pacific Ocean And Inside Many US Cities; via @AGreenRoad
http://agreenroad.blogspot.com/2014/01/fukushima-released-massive-amounts-of.html
Plutonium-238 From Fukushima Traveled Around The World - ‘Misleading’ Experts Said It Would Stay Close By, Or Did Not Happen; via @AGreenRoad
http://agreenroad.blogspot.com/2013/12/plutonium-238-from-fukushima-traveled.html
Plutonium mimics iron in the body, replacing it, and then doing damage where ever it ends up going. Iron (and radioactive plutonium) concentrates in the bone marrow and liver, so that is where most of the damage done by plutonium occurs, but cancers of all kinds are possible, including lung, brain, liver, bone, blood, and more.
End
Is There 'Natural' Plutonium And Are There 'Natural' Fission Reactions in Pitchblende Or Sun? Will Humanity Live Through The Age Of Fission?
http://agreenroad.blogspot.com/2014/01/is-there-natural-plutonium-and-are.html
More articles at;
Individual Radioactive Elements/Isotopes, USA Radiation Exposure Prevention and Reversal, Music
http://agreenroad.blogspot.com/p/individual-radioactive-elementsisotopes.html
(1,000 + Creative Commons Videos)
http://tinyurl.com/agryoutube
A GREEN ROAD PROJECT INDEX
http://tinyurl.com/agrindex
2,000 + Videos And Articles


Post a Comment